INTRODUCTION: The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic raises many questions about the management of patients with significant comorbidities. Hematologic patients are usually fragile due to an important immunosuppression, so the impact of coronavirus disease (COVID-19) is yet to be determined.
MATERIALS AND METHODS: We conducted a single-center retrospective observational study of patients with hematologic malignancies diagnosed with SARS-CoV-2 at Vall d´Hebron University Hospital (HUVH) between March 1st and May 31st 2020 to analyze their clinical characteristics and evolution. Patient's demographic data, underlying pathology, signs and symptoms of COVID-19, treatment received and clinical course were collected. A statistical analysis was performed to identify the possible variables associated with COVID-19 mortality. For this purpose, we used univariate and multivariable logistic regression models.
RESULTS: We identified 70 patients with PCR confirmed SARS-CoV-2 infection and hematologic malignancy. The median age was 75 years (range 22-91), and 44% were female. The majority (74%) had evidence of active malignancy and 53% were receiving active therapy. Lymphoid pathology (73%) predominated over myeloid. The median number of previous lines of treatment was 0 (range 0-6), 23% had received at least 2 lines, whereas 10% underwent hematopoietic stem cell transplantation (HSCT) (5 patients allo-HSCT, 2 auto-HSCT). Half of the patients had more than one pre-existing comorbidity (17% obstructive pulmonary disease).
At diagnosis the most common symptoms were fever (76%), cough (60%) and dyspnea (31%). We observed that 58% of patients presented a chest X-ray compatible with COVID-19. Regarding laboratory parameters, stood out lymphopenia (65% of patients presented <1200 lymphocytes/mm3) and elevation of inflammation parameters, such as D-dimer (median 365 ng/mL, range 50-5860), ferritin (median 1063 ng/mL, range 73-14191), IL-6 (median 59,6 pg/mL, range 3-4079) and PCR (median 11,2 mg/dL, range 0,3-79,9). Empirical therapy for COVID-19 included antibiotics (78%), anti-virals (50%, 3% remdesivir), and hydroxychloroquine (88%). Only 24% received tocilizumab, 50% heparin (33% prophylactic dose), 12% G-CSF, 9% norepinephrine, 4% corticosteroids and 1% ß-IFN. Most of patients (73%) required oxygen therapy: 36% high-flow, 29% low flow and 8% endotracheal intubation. There were 6 patients who did not receive any treatment.
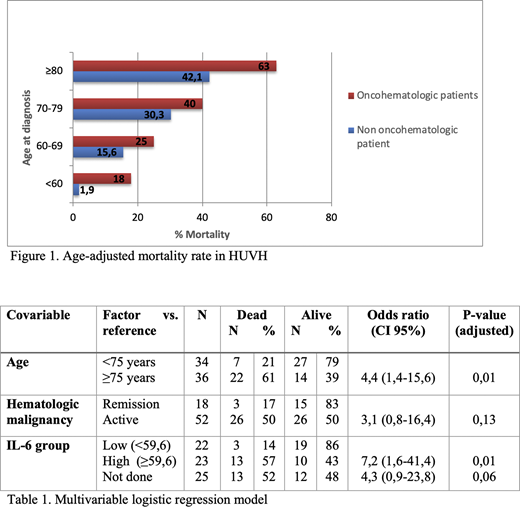
COVID-19 was acquired via nosocomial infection in 23% of patients, 91% of them requiring hospitalization, 14% in the Intensive Care Unit (ICU). The median days of hospitalization since diagnosis was 17 (range 3-55). The case fatality rate (CFR) from COVID-19 was higher in hematologic patients than the one observed in non-hematologic patients at the HUVH (figure 1), being of 41% at 11 days from diagnosis. CFR was higher in patients older than 75 years old (61%), while the mortality among patients receiving active therapy was 42%. The main cause of death was acute respiratory failure (93%). In the univariate logistic regression model, age >75 years (OR 1.07; p=0.008), active malignancy (OR 5; p=0,02), >1 comorbidity (OR 5.3; p=0,049) and high levels of IL-6 (OR 8.2; p= 0.005) were statistically significant. In the multivariable logistic regression model, age ≥75 years (OR 4.4; p=0.01) and IL-6 levels at baseline > 59.6 pg/mL (OR 7.2; p=0.01) were associated with a higher mortality (table 1). The presence of an active malignancy was not a significant variable in the multivariable logistic regression model.
CONCLUSIONS: Patients with hematologic malignancies and COVID-19 presented similar symptoms, signs and radiological characteristics to those described in the general population at diagnosis. In our cohort, advanced age and high IL-6 values were associated with higher mortality. Furthermore, it was observed that active hematologic disease is a factor of poor prognosis of COVID-19.
Salamero:Daichii Sankyo:Honoraria;Celgene:Consultancy, Honoraria;Novartis:Consultancy, Honoraria;Jazz Pharmaceuticals:Consultancy, Honoraria;Pfizer:Consultancy.Abrisqueta:Janssen:Consultancy, Honoraria, Speakers Bureau;AbbVie:Consultancy, Honoraria, Speakers Bureau;Roche:Consultancy, Honoraria, Speakers Bureau;Celgene:Consultancy, Honoraria.Bosch:Hoffmann-La Roche:Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal